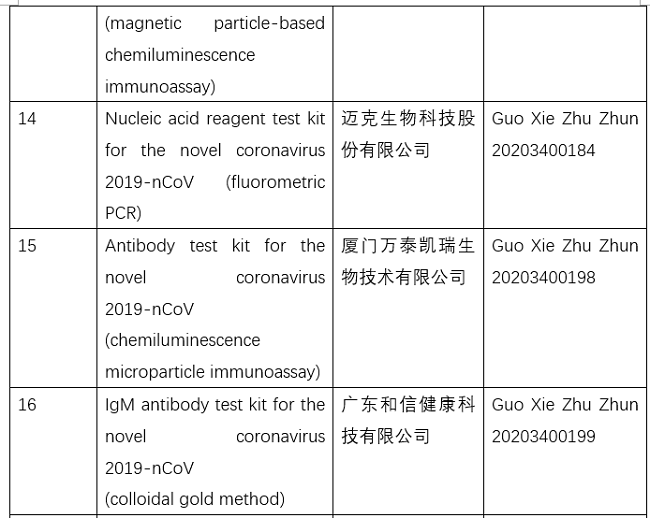
Nmpa Japan. The China National Medical Products Administration or NMPA is the national regulatory body and competent authority for medical devices and in-vitro diagnostics (IVDs) in China. Text is available under the Creative Commons. Understanding the PMDA/NMPA regulatory requirements can be overwhelming and confusing. i Access Consulting assembled a packet that includes the basic information you need before deciding to enter Japan market. Thinking about entering Japan and China markets for the first time? Registration Registration closed There is no registration fee for this seminar. The Ministry of Health, Labour and Welfare (厚生労働省 Kōsei-rōdō-shō?) is a cabinet level ministry of the Japanese government. We provides a wide-range of regulatory services on Medical device, IVD and regenerative medical products registration in Japan PMDA and China NMPA. i Access Consulting is a regulatory consulting company specializing in medical device, IVD and regenerative medical products expansion and compliance for Japan and China markets. Applicants may apply for a drug listing and proceed to conduct the clinical trials while the CDE conducts a technical review of the application materials.
![200GANA-1791 [World Cup watching Nampa! ] Japan national football team ...](https://dutamovie21.pro/wp-content/uploads/2022/11/200GANA-1791-World-Cup-watching-Nampa-768x481.jpg)
Nmpa Japan. The China National Medical Products Administration or NMPA is the national regulatory body and competent authority for medical devices and in-vitro diagnostics (IVDs) in China. This ministry provides regulations on maximum residue limits for agricultural chemicals in foods, basic food and drug regulations, standards for foods, food additives, etc. Registration Registration closed There is no registration fee for this seminar. In China, Pharmaceuticals are regulated by The National Medical Products Administration (NMPA) is the Chinese agency for regulating drugs and medical devices (formerly known as the China Food and Drug Administration or CFDA). The Japan PMDA continues to improve the public health and safety of Japan by reviewing applications for marketing approval of pharmaceuticals and medical devices, conducting safety measures, and providing relief to people who have suffered from adverse drug reactions.. (Chinese and English) to the NMPA request for public comments on the . Nmpa Japan.
The China National Medical Products Administration or NMPA is the national regulatory body and competent authority for medical devices and in-vitro diagnostics (IVDs) in China.
Thinking about entering Japan and China markets for the first time?
Nmpa Japan. We provides a wide-range of regulatory services on Medical device, IVD and regenerative medical products registration in Japan PMDA and China NMPA. i Access Consulting is a regulatory consulting company specializing in medical device, IVD and regenerative medical products expansion and compliance for Japan and China markets. This ministry provides regulations on maximum residue limits for agricultural chemicals in foods, basic food and drug regulations, standards for foods, food additives, etc. Japan, Brazil, Mexico and South Korea. In China, Pharmaceuticals are regulated by The National Medical Products Administration (NMPA) is the Chinese agency for regulating drugs and medical devices (formerly known as the China Food and Drug Administration or CFDA). The NMPA is based in Beijing and overseas the regulation of drugs, medical.
Nmpa Japan.