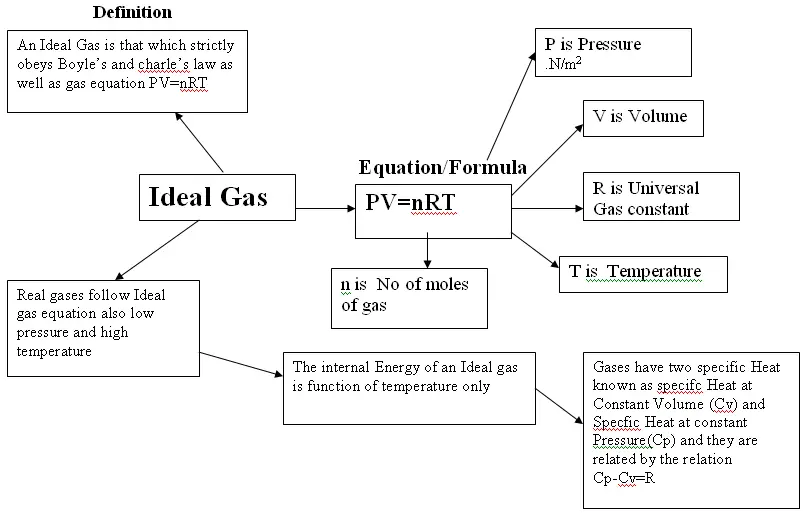
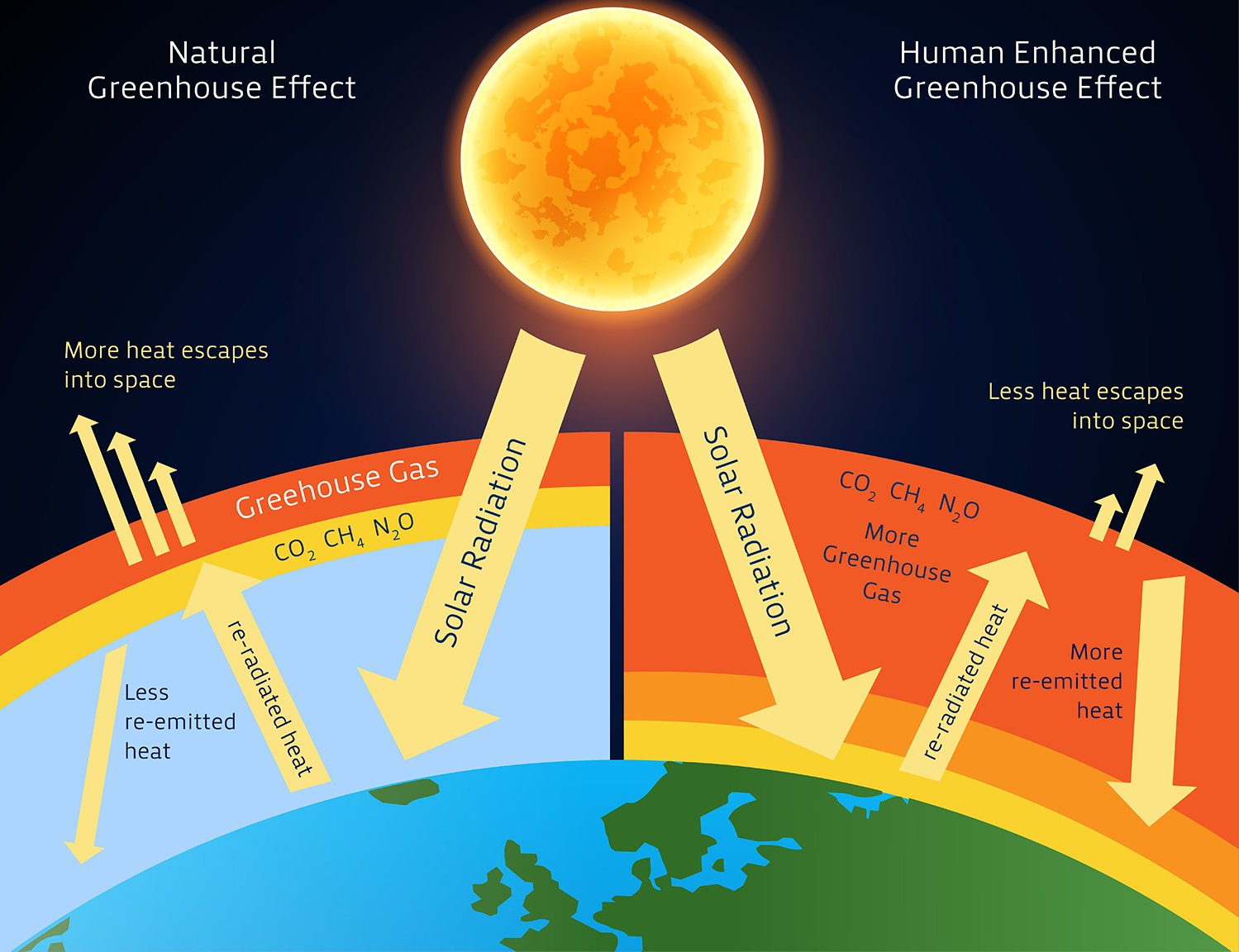
Concept Map About Gases. Structure The remarkable feature of gases is that they appear to have no structure at all. Examples of gases at standard temperature and pressure include: air (a mixture of gases) chlorine at room temperature and pressure. ozone. oxygen. hydrogen. water vapor or steam. As students construct concept maps, they make sense of the ideas and the relationships between them. As the temperature of a gas rises, the average velocity of the molecules will increase; a doubling of the. gas: Kinetic theory of gases. Molar volume of a gas: standard. We live in a gaseous atmosphere and are influenced by its composition. But for the purposes of this course, it is vital for appreciating the limitations of the scientific model that consistutes the "ideal gas". gases and proposing a model to explain an everyday change of state—boiling. According to the kinetic molecular theory, the average kinetic energy of an ideal gas is directly proportional to the absolute temperature.

Concept Map About Gases. The flow is as follows: Respirations Inhalation of air Expiration to atmosphere Alveoli Pulmonary capillaries Diffusion Hemoglobin carrying capacity Perfusion Delivery of oxygen and nutrients Cells lining alveoli Cellular uptake of oxygen and nutrients Concept Map. Here's a basic structure for your concept map: Gas Exchange; Definition: The process by which oxygen is taken in and carbon dioxide is expelled out. Energy, light, and sound, however, are not matter; ideas and emotions are also not matter. Introduction The three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. The molecules are rigid and perfectly elastic spheres of very small diameters. Concept Map About Gases.
Volume (L, M, ML, Cm) The kinetic theory of gases is developed with certain assumptions about the nature and state of motion of the molecules of gases.
As students construct concept maps, they make sense of the ideas and the relationships between them.
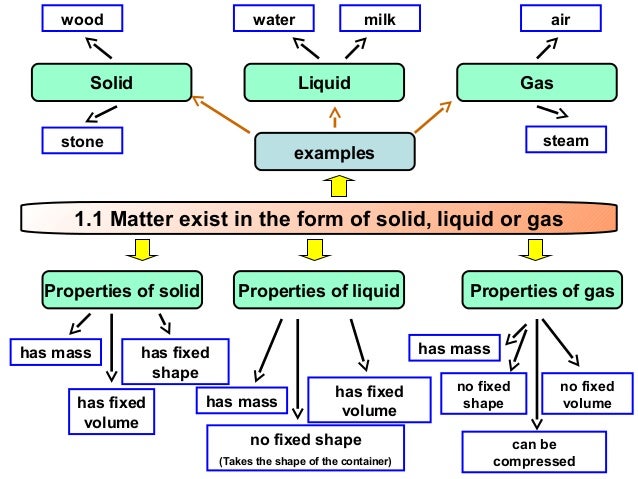
Concept Map About Gases. States of matter concept map showing the classical states along with two other states known as plasma and Bose-Einstein condensate. Gold and iridium are matter, as are peanuts, people, and postage stamps. The flow is as follows: Respirations. It is of obvious interest to a chemical engineer who needs to predict the properties of the gases involved in a chemical reaction carried out at several hundred atmospheres pressure. Gases will have low density as they occupy large volume for very little mass.
Concept Map About Gases.